- Ni(dmg)2 Structure
- Ni Dmg 2 2 Structure System
- Ni Dmg 2 2 Structures
- Nickel Dimethylglyoxime
- Ni Dmg 2 2 Structure Diagram
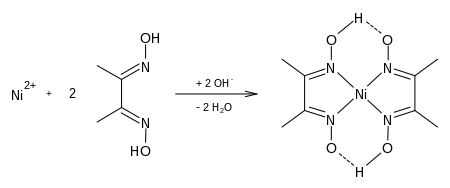
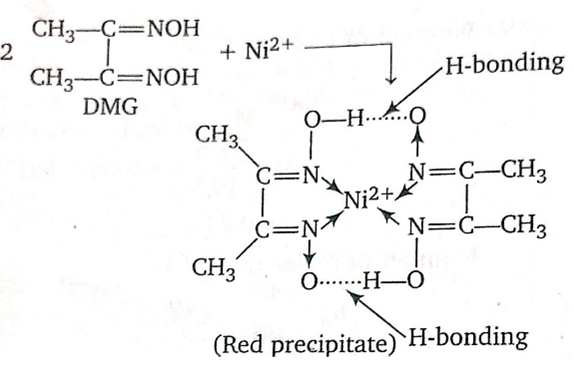
Given The Structure Of Ni(dmgH)2, Do You Think DmgH Is A Strong Or Weak Field Ligand? Given The Structure Of Is Ni(acac)2(H2O)2, Do You Think Acac- Is A Strong Or Weak Field Ligand? (2 Marks) This problem has been solved! See the answer. Show transcribed image text. Nov 16, 2010 here is the structure of nickel DMG. U can calculate the molecualr formula and then divide it by atomic mass of nickel and multiply by 100 to get your answer. The molar mass of Ni(DMGH)2 is 58.7+117.2.2=293.1. So% of nickel = 100.58.7/293.1 =34.1%. Login to reply the answers Post; Still have questions? Get your answers by asking now. Ni(DMG)2 results are thoroughly compared with Cu(DMG)2 and also against available experimental data. Stronger H-bonding leads to greater stability of Ni(DMG)2 with respect to isolated ions (M2+ and DMG–) compared to Cu(DMG)2. Structural, Electronic, and Spectral Properties of Metal Dimethylglyoximato M(DMG) 2; M = Ni 2+, Cu 2+ Complexes: A Comparative Theoretical Study October 2019 The Journal of Physical.
2 + 2 dmg All of the complexes are Ni2+octahedral except from Ni(CN) 4 2-which is a Ni2+ square planer complex. As the strength of the field ligand increases the field splitting increase. The ligands, used in this demonstration, listed in order of increasing field strength are.
| Names | |
|---|---|
| IUPAC name | |
| Other names Nickel hydroxide, Theophrastite | |
| Identifiers | |
| |
| ChemSpider | |
| ECHA InfoCard | 100.031.813 |
| EC Number |
|
| RTECS number | |
CompTox Dashboard(EPA) | |
| |
| |
| Properties | |
| Ni(OH)2 | |
| Molar mass | 92.724 g/mol (anhydrous) 110.72 g/mol (monohydrate) |
| Appearance | green crystals |
| Density | 4.10 g/cm3 |
| Melting point | 230 °C (446 °F; 503 K) (anhydrous, decomposes) |
| 0.13 g/L | |
| +4500.0·10−6 cm3/mol | |
| Structure[1] | |
| hexagonal, hP3 | |
| P3m1, No. 164 | |
α = 90°, β = 90°, γ = 120° | |
| Thermochemistry | |
| 79 J·mol−1·K−1[2] | |
Std enthalpy of formation(ΔfH⦵298) | −538 kJ·mol−1[2] |
| Hazards | |
| Safety data sheet | External SDS |
| GHS pictograms | [3] |
| GHS Signal word | Danger[3] |
| H302, H332, H315, H334, H317, H341, H350, H360, H372[3] | |
| P260, P284, P201, P280, P405, P501[3] | |
| Lethal dose or concentration (LD, LC): | |
| 1515 mg/kg (oral, rat) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| verify (what is ?) | |
| Infobox references | |
Ni(dmg)2 Structure
Nickel(II) hydroxide is the inorganic compound with the formula Ni(OH)2. It is an apple-green solid that dissolves with decomposition in ammonia and amines and is attacked by acids. It is electroactive, being converted to the Ni(III) oxy-hydroxide, leading to widespread applications in rechargeable batteries.[4]
Properties[edit]
Nickel(II) hydroxide has two well-characterized polymorphs, α and β. The α structure consists of Ni(OH)2 layers with intercalated anions or water.[5][6] The β form adopts a hexagonal close-packed structure of Ni2+ and OH− ions.[5][6] In the presence of water, the α polymorph typically recrystallizes to the β form.[5][7] In addition to the α and β polymorphs, several γ nickel hydroxides have been described, distinguished by crystal structures with much larger inter-sheet distances.[5]
The mineral form of Ni(OH)2, theophrastite, was first identified in the Vermion region of northern Greece, in 1980. It is found naturally as a translucent emerald-green crystal formed in thin sheets near the boundaries of idocrase or chlorite crystals.[8] A nickel-magnesium variant of the mineral, (Ni,Mg)(OH)2 had been previously discovered at Hagdale on the island of Unst in Scotland.[9]
Reactions[edit]
Nickel(II) hydroxide is frequently used in electrical car batteries.[6] Specifically, Ni(OH)2 readily oxidizes to nickel oxyhydroxide, NiOOH, in combination with a reduction reaction, often of a metal hydride (reaction 1 and 2).[10]
Reaction 1 Ni(OH)2 + OH− → NiO(OH) + H2O + e−
Reaction 2 M + H2O + e− → MH + OH−
Net Reaction (in H2O)Ni(OH)2 + M → NiOOH + MH
Of the two polymorphs, α-Ni(OH)2 has a higher theoretical capacity and thus is generally considered to be preferable in electrochemical applications. However, it transforms to β-Ni(OH)2 in alkaline solutions, leading to many investigations into the possibility of stabilized α-Ni(OH)2 electrodes for industrial applications.[7]
Synthesis[edit]
The synthesis entails treating aqueous solutions of nickel(II) salts with potassium hydroxide.[11]
Toxicity[edit]
The Ni2+ ion is a known carcinogen. Toxicity and related safety concerns have driven research into increasing the energy density of Ni(OH)2 electrodes, such as the addition of calcium or cobalt hydroxides.[4]
Ni Dmg 2 2 Structure System
See also[edit]
References[edit]
Ni Dmg 2 2 Structures
- ^Enoki, Toshiaki; Tsujikawa, Ikuji (1975). 'Magnetic Behaviours of a Random Magnet, NipMg(1-p)(OH2)'. Journal of the Physical Society of Japan. 39 (2): 317. doi:10.1143/JPSJ.39.317.
- ^ abZumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN978-0-618-94690-7.
- ^ abcd'Nickel Hydroxide'. American Elements. Retrieved 2018-08-30.
- ^ abChen, J.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. (1999). 'Nickel Hydroxide as an Active Material for the Positive Electrode in Rechargeable Alkaline Batteries'. J. Electrochem. Soc. 146 (10): 3606–3612. doi:10.1149/1.1392522.
- ^ abcdOliva, P.; Leonardi, J.; Laurent, J.F. (1982). 'Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides'. Journal of Power Sources. 8 (2): 229–255. doi:10.1016/0378-7753(82)80057-8.
- ^ abcJeevanandam, P.; Koltypin, Y.; Gedanken, A. (2001). 'Synthesis of Nanosized α-Nickel Hydroxide by a Sonochemical Method'. Nano Letters. 1 (5): 263–266. doi:10.1021/nl010003p.
- ^ abShukla, A.K.; Kumar, V.G.; Munichandriah, N. (1994). 'Stabilized α-Ni(OH)2 as Electrode Material for Alkaline Secondary Cells'. J. Electrochem. Soc. 141 (11): 2956–2959. doi:10.1149/1.2059264.
- ^Marcopoulos, T.; Economou, M. (1980). 'Theophrastite, Ni(OH)2, a new mineral from northern Greece'(PDF). American Mineralogist. 66: 1020–1021.
- ^Livingston, A.; Bish, D. L. (1982). 'On the new mineral theophrastite, a nickel hydroxide, from Unst, Shetland, Scotland'(PDF). Mineralogical Magazine. 46 (338): 1. doi:10.1180/minmag.1982.046.338.01.
- ^Ovshinsky, S.R.; Fetcenko, M.A.; Ross, J. (1993). 'A nickel metal hydride battery for electric vehicles'. Science. 260 (5105): 176–181. doi:10.1126/science.260.5105.176. PMID17807176.
- ^Glemser, O. (1963) 'Nickel(II) Hydroxide' in 'Handbook of Preparative Inorganic Chemistry, 2nd ed. G. Brauer (ed.), Academic Press, NY. Vol. 1. p. 1549.
Comments are closed.